


Propagate Success with Clean Suckers
The propagation of disease-cleaned suckers involves two main techniques: field-based and detached corm methods. Field-based techniques include complete and false decapitation, stimulating lateral production of sucker buds by destroying the meristematic corm. Partial decapitation involves making a hole in the pseudostem to destroy the meristem. Detached corm techniques use excised buds or plants resulting from stem fragments, producing a higher number of seedlings with greater growth uniformity. It's crucial that starting material for macro-propagation is free of pests and diseases. Clean knives and hardened sprouts are essential for all methods to prevent disease transmission. Additionally, a propagation chamber of specific dimensions is used, filled with a substrate mixture sterilized by steam to ensure optimal conditions for growth.
This technology is TAAT1 validated.
Adults 18 and over: Positive high
The poor: Positive high
Under 18: Positive medium
Women: Positive high
Climate adaptability: Highly adaptable
Farmer climate change readiness: Significant improvement
Biodiversity: Positive impact on biodiversity
Carbon footprint: A bit less carbon released
Environmental health: Greatly improves environmental health
Soil quality: Improves soil health and fertility
Water use: Much less water used
In the near future, this section will provide an overview of this technology's success in various contexts, details on partners offering technical support, training, and implementation monitoring, along with other valuable insights for your projects and programs. These details will be added progressively.
In the meantime, use the 'Request information' button if you need to contact us.
2,500 plantlets shade house
Cost of chamber of 8,000 plantlets
Open source / open access
Scaling Readiness describes how complete a technology’s development is and its ability to be scaled. It produces a score that measures a technology’s readiness along two axes: the level of maturity of the idea itself, and the level to which the technology has been used so far.
Each axis goes from 0 to 9 where 9 is the “ready-to-scale” status. For each technology profile in the e-catalogs we have documented the scaling readiness status from evidence given by the technology providers. The e-catalogs only showcase technologies for which the scaling readiness score is at least 8 for maturity of the idea and 7 for the level of use.
The graph below represents visually the scaling readiness status for this technology, you can see the label of each level by hovering your mouse cursor on the number.
Read more about scaling readiness ›
Uncontrolled environment: tested
Used by some intended users, in the real world
| Maturity of the idea | Level of use | |||||||||
| 9 | ||||||||||
| 8 | ||||||||||
| 7 | ||||||||||
| 6 | ||||||||||
| 5 | ||||||||||
| 4 | ||||||||||
| 3 | ||||||||||
| 2 | ||||||||||
| 1 | ||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
| Country | Testing ongoing | Tested | Adopted |
|---|---|---|---|
| Benin | –No ongoing testing | Tested | –Not adopted |
| Burkina Faso | –No ongoing testing | Tested | –Not adopted |
| Burundi | –No ongoing testing | Tested | –Not adopted |
| Cameroon | –No ongoing testing | Tested | –Not adopted |
| Côte d’Ivoire | –No ongoing testing | Tested | –Not adopted |
| Democratic Republic of the Congo | –No ongoing testing | Tested | –Not adopted |
| Ethiopia | –No ongoing testing | Tested | –Not adopted |
| Ghana | –No ongoing testing | Tested | –Not adopted |
| Kenya | –No ongoing testing | Tested | –Not adopted |
| Malawi | –No ongoing testing | Tested | –Not adopted |
| Mali | –No ongoing testing | Tested | –Not adopted |
| Nigeria | –No ongoing testing | Tested | –Not adopted |
| Rwanda | –No ongoing testing | Tested | Adopted |
| Somalia | –No ongoing testing | Tested | –Not adopted |
| South Sudan | –No ongoing testing | Tested | –Not adopted |
| Tanzania | –No ongoing testing | Tested | Adopted |
| Togo | –No ongoing testing | Tested | –Not adopted |
| Uganda | –No ongoing testing | Tested | Adopted |
| Zambia | –No ongoing testing | Tested | –Not adopted |
This technology can be used in the colored agro-ecological zones. Any zones shown in white are not suitable for this technology.
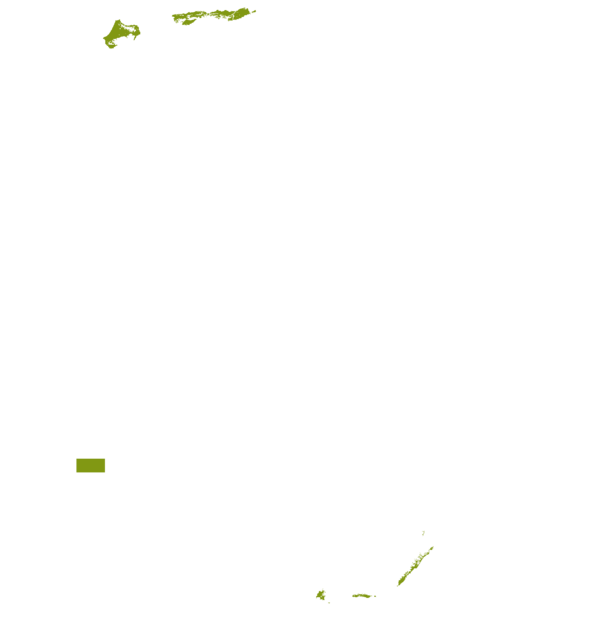
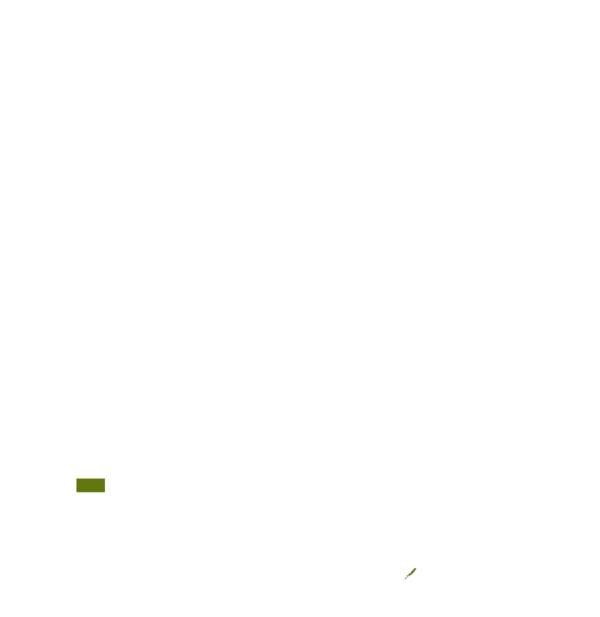
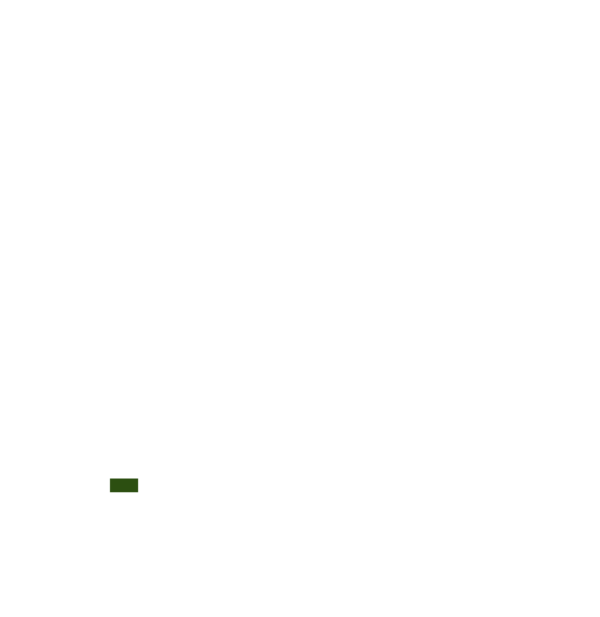
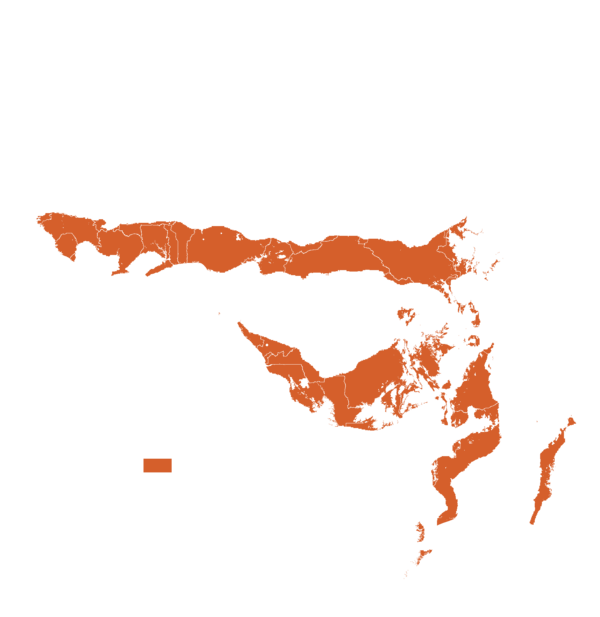

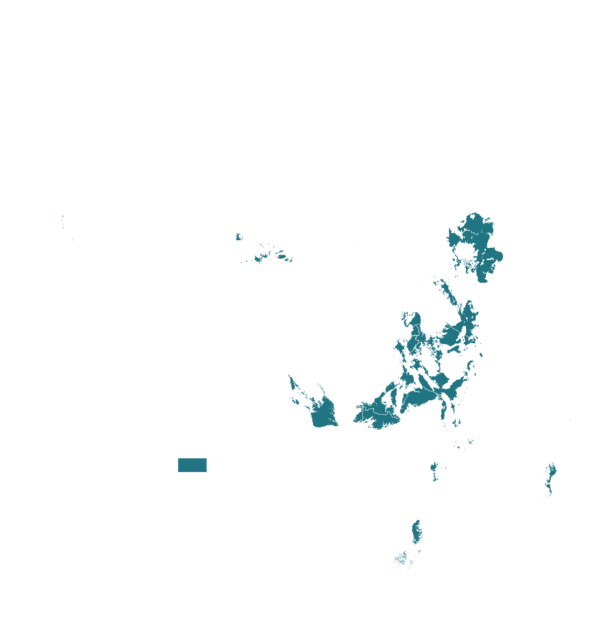
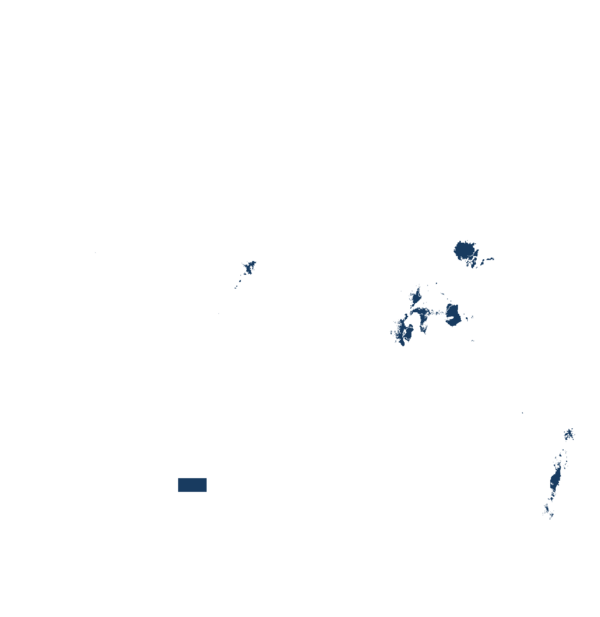
| AEZ | Subtropic - warm | Subtropic - cool | Tropic - warm | Tropic - cool |
|---|---|---|---|---|
| Arid | – | – | – | – |
| Semiarid | – | – | – | – |
| Subhumid | ||||
| Humid |
Source: HarvestChoice/IFPRI 2009
The United Nations Sustainable Development Goals that are applicable to this technology.



False Decapitation:
a. Select a 6-month-old banana or plantain plant.
b. Incise the pseudostem at 20 cm from the ground level, creating a square hole.
c. Angle the bottom side of the hole slightly downwards to collect water and sap.
d. Leave the decapitated plant for at least one month to allow for sprouting.
e. After sprouting, detach suckers with three to four leaves and transplant them to the field.
Complete Decapitation:
a. Choose a 6-month-old plant.
b. Cut down the plant to ground level.
c. Excise the middle 5 cm of the softer meristem, leaving the harder corm intact.
d. Cover the cut stem with soil to promote sprouting.
e. Within three weeks, four to seven suckers will emerge.
f. Detach suckers with three to four leaves and transplant them to the field.
Detached Corm Technique:
a. Collect healthy suckers between flowering and harvest.
b. Cut roots from the suckers and wash them.
c. Peel the leaf sheaths from the suckers.
d. Submerge the whole corm in boiling water for 30-40 minutes or use fungicide for 20 minutes to sanitize.
e. Scarify the corm by making a shallow incision at the top.
f. Let the corm air dry for 24 hours.
g. Plant the whole corm in the weaning chamber at a 30 cm distance or split it into 2 or 3 fragments/buds and plant at a 10 cm distance.
h. Cover with 2 cm of sawdust.
Weaning Chamber Preparation:
a. Create a propagation chamber with dimensions 1.5 m wide, 5 m long, and 1 m high.
b. Cover it with transparent polyethylene, ensuring at least 50% shading.
c. Fit plastic covers to maintain high humidity and temperature.
d. Fill the chamber with a substrate mixture of soil, composted manure, and sawdust or other materials at a ratio of 6:3:1.
e. Steam-sterilize the substrate by placing it on top of a metal drum containing boiling water.
Knife Sterilization:
a. Clean knives with boiled water before use to avoid disease transmission.
b. For the detached corm technique, ensure that knives are specifically cleaned when using hardened sprouts.
Propagation Unit Setup:
a. Determine locations for macro-propagation units and nurseries.
b. Develop a timeline for unit setup.
c. Mobilize materials and resources for unit setup.
d. Specify activities involved in setting up and maintaining the units.
e. Establish a plan for sharing/distributing planting material.
f. Ensure financial and environmental sustainability of the unit.
Last updated on 31 October 2025