

A rapid quality plantlets delivery technology for banana
In-vitro micro-propagation is a plant propagation technique conducted under controlled laboratory conditions, involving the multiplication of plant tissue through distinct stages. Beginning with the initiation of aseptic plant material, typically from meristematic tissue, in a sterile culture medium, the process progresses through stages of rapid multiplication, rooting, and hardening. This method offers several advantages, including disease elimination, fast multiplication of uniform plantlets, genetic preservation, and uniformity in growth characteristics. However, successful implementation requires substantial investment in laboratory infrastructure and skilled personnel, strict adherence to aseptic standards, and careful handling of delicate plantlets throughout the process. Despite these challenges, in-vitro micro-propagation stands as a valuable technique for producing healthy, uniform, and genetically consistent planting material, contributing to improved crop productivity and quality.
This technology is TAAT1 validated.
Positive or neutral impact
Positive or neutral impact
ORG specific text
In the meantime, use the “Request information” button if you need to contact us.
Per plantlets
Profit
A nursery business can produce 3,000 TC plantlets per cycle
No formal IP rights
| Country | Tested | Adopted |
|---|---|---|
| Burundi | –Not tested | Adopted |
| Cameroon | –Not tested | Adopted |
| Côte d’Ivoire | –Not tested | Adopted |
| Democratic Republic of the Congo | –Not tested | Adopted |
| Ethiopia | –Not tested | Adopted |
| Ghana | –Not tested | Adopted |
| Kenya | –Not tested | Adopted |
| Nigeria | –Not tested | Adopted |
| Rwanda | –Not tested | Adopted |
| Somalia | –Not tested | Adopted |
| Tanzania | –Not tested | Adopted |
| Uganda | –Not tested | Adopted |
| Zambia | –Not tested | Adopted |
This technology can be used in the colored agro-ecological zones. Any zones shown in white are not suitable for this technology.
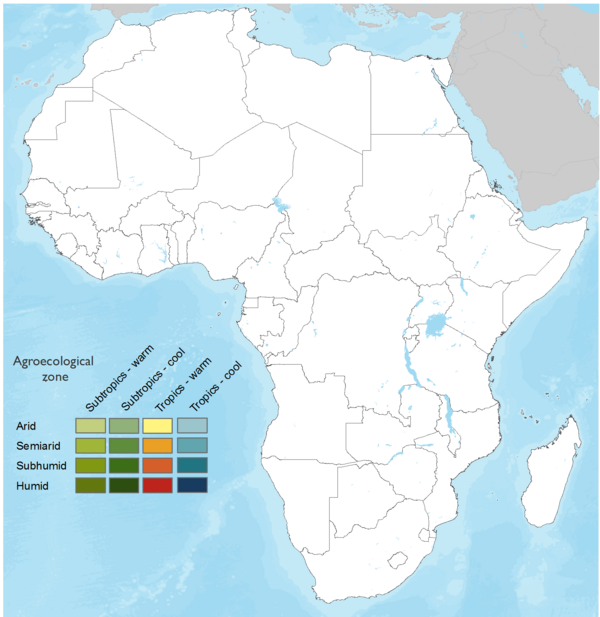
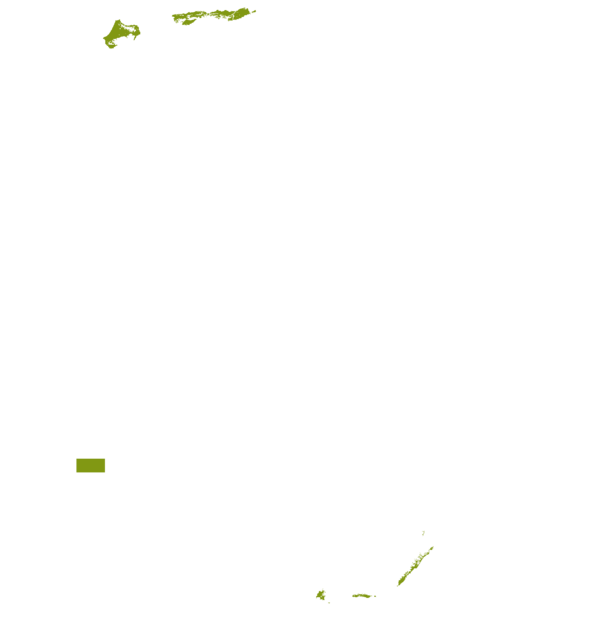
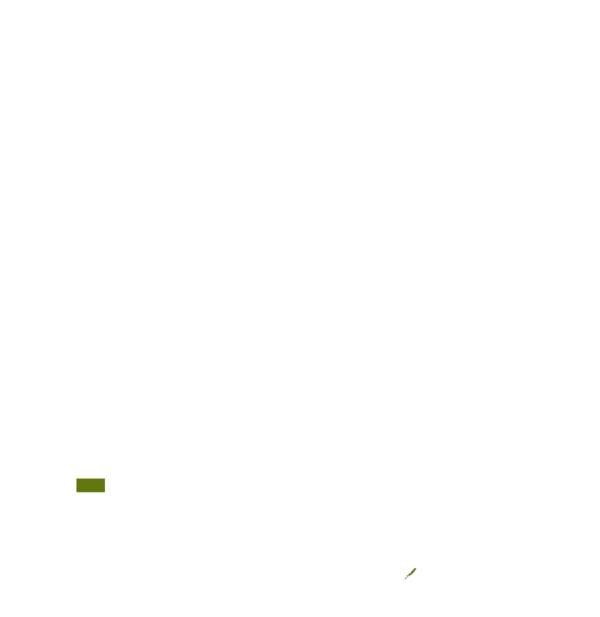
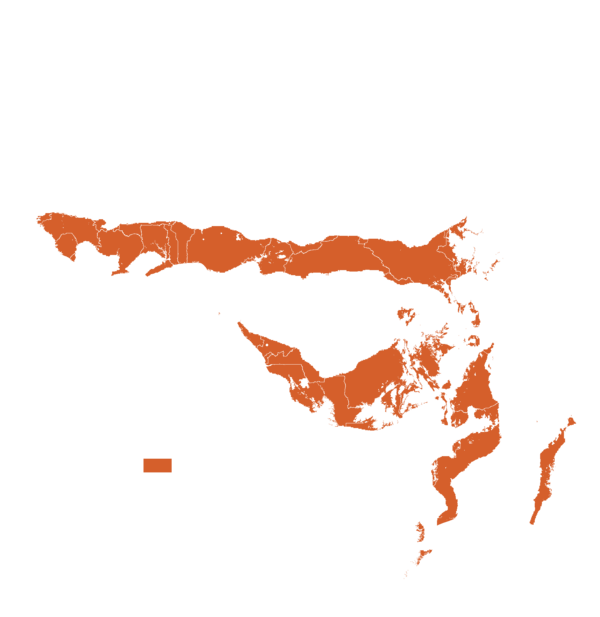

| AEZ | Subtropic - warm | Subtropic - cool | Tropic - warm | Tropic - cool |
|---|---|---|---|---|
| Arid | – | – | – | – |
| Semiarid | – | – | – | – |
| Subhumid | – | – | ||
| Humid | – | – |
Source: HarvestChoice/IFPRI 2009
The United Nations Sustainable Development Goals that are applicable to this technology.


The application of this technology involves several steps.
Collection of Disease-Free Suckers:
Meristematic Tissue Removal:
Corm Sterilization:
Corm Trimming:
Corm Cutting into Propagules:
Placement in Tubes with Sterile Growth Medium:
Growth Progress Monitoring:
Transfer to Jars for Shoot Growth:
Growth Chamber Placement:
3-4 Weeks of Growth:
Last updated on 22 May 2024