

Virus diagnostic tool for cassava seed health certification by seed producers and seed certifiers.
Cassava virus indexing is a technical process to detect and eliminate virus-infected cassava planting materials from the seed value chain, especially at the nucleus and early generation seed production stages. Virus indexing involves testing plants using sensitive diagnostic methods like Polymerase Chain Reaction (PCR) and isothermal amplification [e.g., Loop-mediated Isothermal Amplification (LAMP) ]to identify infected materials. Only virus-free plants are selected for early-generation seed production. This process safeguards seed quality, enhances crop resilience, and is essential for effective seed certification systems, supporting food security in cassava-growing regions. This technology is critical for nucleus and EGS cassava seed producers, as well as cassava seed regulatory (seed certifiers) for diagnostics-based certification of EGS fields (breeder and foundation seed).
This technology is pre-validated.
Adults 18 and over: No impact
Not applicable as technology is neutral to age and gender
The poor: Positive high
The technology helps prevent the introduction of invasive viruses and protect biodiversity and landraces.
Under 18: No impact
Not applicable as technology is neutral to age and gender; although not relevant to group under 18 years.
Women: No impact
Not applicable as technology is neutral to age and gender
Climate adaptability: Highly adaptable
As a laboratory tool, adoptable and usable under all conditions.
Farmer climate change readiness: Significant improvement
The technology reduces the risk of vector-borne viruses in the field, which are expected to increase under changing climate conditions.
Biodiversity: Positive impact on biodiversity
The technology helps prevention of introduction of invasive viruses and protect biodiversity and landraces
Cassava Virus Indexing technology helps tackle major challenges in seed quality and virus spread in cassava production. By detecting and removing virus-infected planting materials early, it protects cassava yields, improves seed certification systems, and supports food security in cassava-growing regions—aligning with global sustainability and resilience goals.
To successfully integrate this technology into your national or regional projects, consider the following steps:
Cost per sample for testing
No formal IP rights
Scaling Readiness describes how complete a technology’s development is and its ability to be scaled. It produces a score that measures a technology’s readiness along two axes: the level of maturity of the idea itself, and the level to which the technology has been used so far.
Each axis goes from 0 to 9 where 9 is the “ready-to-scale” status. For each technology profile in the e-catalogs we have documented the scaling readiness status from evidence given by the technology providers. The e-catalogs only showcase technologies for which the scaling readiness score is at least 8 for maturity of the idea and 7 for the level of use.
The graph below represents visually the scaling readiness status for this technology, you can see the label of each level by hovering your mouse cursor on the number.
Read more about scaling readiness ›
Uncontrolled environment: validated
Used by some intended users, in the real world
| Maturity of the idea | Level of use | |||||||||
| 9 | ||||||||||
| 8 | ||||||||||
| 7 | ||||||||||
| 6 | ||||||||||
| 5 | ||||||||||
| 4 | ||||||||||
| 3 | ||||||||||
| 2 | ||||||||||
| 1 | ||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
| Country | Testing ongoing | Tested | Adopted |
|---|---|---|---|
| Benin | –No ongoing testing | Tested | –Not adopted |
| Burundi | –No ongoing testing | –Not tested | Adopted |
| Côte d’Ivoire | –No ongoing testing | Tested | –Not adopted |
| Democratic Republic of the Congo | –No ongoing testing | –Not tested | Adopted |
| Ghana | –No ongoing testing | Tested | –Not adopted |
| Kenya | –No ongoing testing | –Not tested | Adopted |
| Malawi | –No ongoing testing | Tested | –Not adopted |
| Nigeria | –No ongoing testing | –Not tested | Adopted |
| Sierra Leone | –No ongoing testing | Tested | –Not adopted |
| Tanzania | –No ongoing testing | –Not tested | Adopted |
| Uganda | –No ongoing testing | –Not tested | Adopted |
| Zambia | –No ongoing testing | Tested | –Not adopted |
This technology can be used in the colored agro-ecological zones. Any zones shown in white are not suitable for this technology.
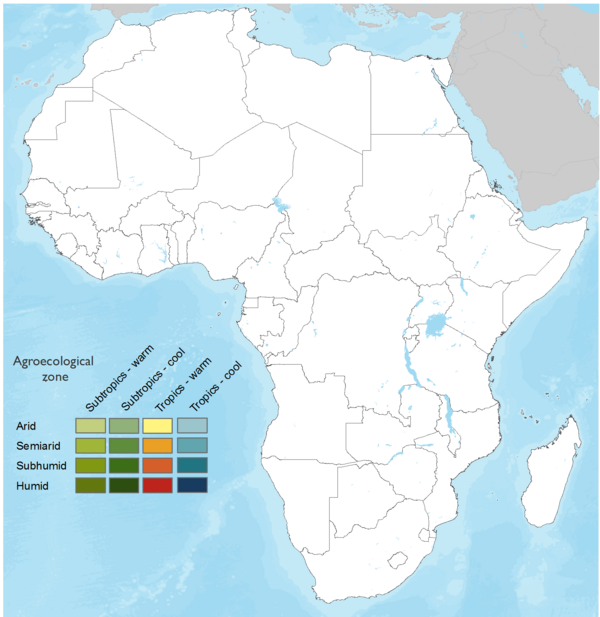
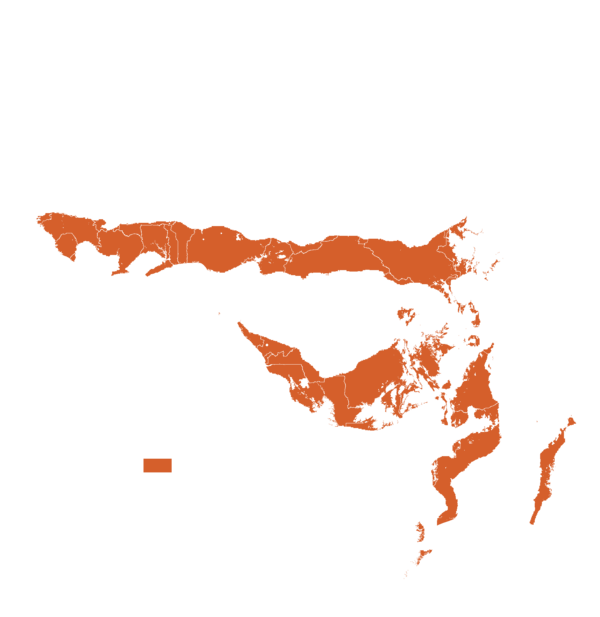

| AEZ | Subtropic - warm | Subtropic - cool | Tropic - warm | Tropic - cool |
|---|---|---|---|---|
| Arid | – | – | – | – |
| Semiarid | – | – | – | – |
| Subhumid | – | – | – | |
| Humid | – | – | – |
Source: HarvestChoice/IFPRI 2009
The United Nations Sustainable Development Goals that are applicable to this technology.

End hunger, achieve food security, and promote sustainable agriculture

Ensure healthy lives through improved crop health

Ensure sustainable consumption and production patterns
Set Up a Diagnostic Lab:
Equip the lab with necessary tools, including nucleic acid extraction kits, thermal cyclers, and reagents for PCR and LAMP tests.
Collect Plant Samples:
Sample cassava plants during early-generation seed production using a standardized protocol.
Virus Detection:
Use PCR or LAMP methods to test for viruses like CMD and CBSD in the plant tissue.
Select Virus-Free Plants:
Only select plants that test negative for viruses for early-generation seed production.
Train Personnel:
Train lab and field staff on virus testing, equipment use, and result interpretation.
Standardize Protocols:
Follow consistent protocols for sampling, testing, and interpreting results to ensure reliable outcomes.
Ensure Seed Integrity:
Regularly test seeds to maintain virus-free stock and update testing procedures as needed.
Integrate into Certification:
Use virus indexing results as part of the seed certification process for virus-free early-generation seeds.
Last updated on 28 November 2025